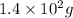Answer:
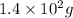
Step-by-step explanation:
Depression in freezing point is given by:
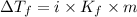
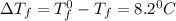 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor = 1 (for non electrolyte like glycine)
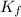 = freezing point constant = ?
= freezing point constant = ?
m= molality
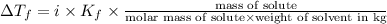
Weight of solvent = 950 g = 0.95 kg
Molar mass of glycine = 75.07 g/mol
Mass of glycine added = 282 g
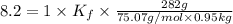
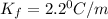
Thus freezing point constant is
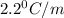
2)
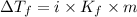
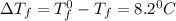 = Depression in freezing point
= Depression in freezing point
i= vant hoff factor = 4 (for
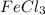 )
)
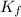 = freezing point constant =
= freezing point constant =
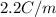
m= molality
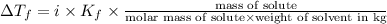
Weight of solvent = 950 g = 0.95 kg
Molar mass of
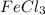 = 162.2 g/mol
= 162.2 g/mol
Mass of
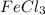 added = ?
added = ?
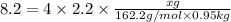
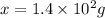
Thus mass of iron(III) chloride that must be dissolved in the same mass of to produce the same depression in freezing point is