Answer:
55.3 M
Step-by-step explanation:
Given temperature = 25°C
Density = 0.997 g/mL
Based on density the mass of water per L = 997 grams
The molar mass of water = 18
The moles of water =
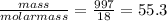
The molarity is defined as the moles of water per litre.
The moles = 55.3
Volume = 1 L
So molarity = 55.3 /1 = 55.3 M