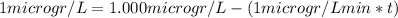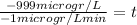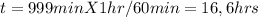Answer:
It would be required 16.6 hours in the bioreactor for reduce the TCA concentration from 1 mg/L to 1 µg/L in the effluent.
Step-by-step explanation:
A zero order reaction is a chemical reaction in which the rate of reaction is constant and independent of the concentration of the reacting substances, as is assumed in this problem with the biodegradation rate for TCA:
Zero order reaction formula:
![[A] =[A]_(0) - kt](https://img.qammunity.org/2020/formulas/biology/college/rl7im697tz5yvlble470nef9ms6dujx6mp.png)
Where
![[A]](https://img.qammunity.org/2020/formulas/chemistry/college/ey5pxctwpmy356s81f6qc2pjl6oveugako.png) and
and
![[A]_(0)](https://img.qammunity.org/2020/formulas/chemistry/college/qqxha3gkqyi6nvxca57jmthzzzu8zyjq3q.png) are TCA concentrations, 1 µg/L and 1 mg/L respectively.
are TCA concentrations, 1 µg/L and 1 mg/L respectively.
t = time of reaction
k is a constant, in this case is the rate for TCA removal = 1 µg/Lmin
1 mg/l = 1000µg/L
![[A] =[A]_(0) - kt](https://img.qammunity.org/2020/formulas/biology/college/rl7im697tz5yvlble470nef9ms6dujx6mp.png)
Replacing