Answer:
Aluminum
Step-by-step explanation:
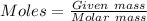
Mass of
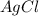 = 1.68 g
= 1.68 g
Molar mass of
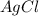 = 143.32 g/mol
= 143.32 g/mol
Thus,
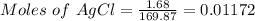
From the reaction below:
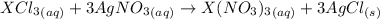
3 moles of
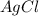 are produced when 1 mole of
are produced when 1 mole of
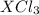 undergoes reaction.
undergoes reaction.
So,
1 mole of
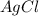 are produced when
are produced when
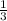 mole of
mole of
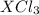 undergoes reaction.
undergoes reaction.
0.01172 mole of
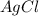 are produced when
are produced when
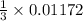 mole of
mole of
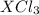 undergoes reaction.
undergoes reaction.
Thus, moles of
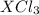 = 0.0039 moles
= 0.0039 moles
Let the atomic mass of X = x g/mol
atomic mass of chlorine = 35.5 g/mol
Thus, Molar mass of
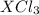 = x + 3(35.5) g/mol = x + 106.5 g/mol
= x + 3(35.5) g/mol = x + 106.5 g/mol
Moles = 0.0039 moles
Mass = 0.521 g
Thus, molar mass = Given mass/ Moles = 0.521 / 0.0039 = 133.5897 g/mol
So,
x + 106.5 = 133.5897
x = 27.0897 g/mol
This Atomic weight corresponds to Aluminum. Hence, X is aluminum.