Answer:
Just above of 3000
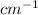
Step-by-step explanation:
The strength of C-H bond determines the position of the C-H stretching vibration peak higher strength leads to higher wavenumber.
C atoms sp hybridized (triple bond between carbon atoms) have more sigma character in their bonds (there are less p orbitals involved), hence their bonds are stronger. The C-H stretching of sp hybridized carbon are around 3300
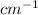 .
.
C atoms sp
 hybridized (single bond between carbon atoms) have less sigma character in their bonds (there are more p orbitals involved), hence their bond are weaker. The C-H stretching of C sp
hybridized (single bond between carbon atoms) have less sigma character in their bonds (there are more p orbitals involved), hence their bond are weaker. The C-H stretching of C sp
 hybridized are around 2900
hybridized are around 2900
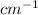 .
.
C atoms sp
 hybridized (double bond between carbon atoms) have less sigma character than sp atoms, but more sigma character than sp
hybridized (double bond between carbon atoms) have less sigma character than sp atoms, but more sigma character than sp
 atoms in their bonds. Hence their bonds have intermediate strength. The C-H stretching of C sp
atoms in their bonds. Hence their bonds have intermediate strength. The C-H stretching of C sp
 hybridized are around 3100
hybridized are around 3100
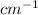 .
.