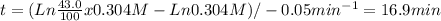Answer:
It take 16.9 min for the concentration of A to decrease by 43.0 %
Step-by-step explanation:
The first-order reaction law is
![Ln [A] = -k.t + Ln [A]_(0)](https://img.qammunity.org/2020/formulas/chemistry/high-school/b4qlnkdz5z0nhm4wmo9fk52a2zoc4d0qwo.png)
And the half-life time for the first-order reaction is
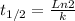
- [A] is the concentration of the reactant at any time (t) of the reaction
- [A]0 is the concentration of the reactant at the beginning of the reaction
- k is the rate constant.
- t1/2 is the half-life time
Thus, for this reaction k is
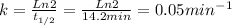
Decreasing the concentration of the reactant by 43.0% means the concentration of A (reactant) at the end of the reaction has to be:
![[A] = (43.0)/(100)x[A]_(0)](https://img.qammunity.org/2020/formulas/chemistry/high-school/s9i5f0p9ozz030q9u7l8pgzsxpcy6xnwtg.png)
Replacing [A] in the equation of the law
![Ln (43.0)/(100) [A]_(0) = -k.t + Ln [A]_(0)](https://img.qammunity.org/2020/formulas/chemistry/high-school/w26feq109teymdybllyq35w8x92otd5igb.png)
Clearing the t
![(Ln (43.0)/(100) [A]_(0) - Ln [A]_(0) ) / -k = t](https://img.qammunity.org/2020/formulas/chemistry/high-school/wko56wlp0mlrpu94xxswjwbjp2pb2hp7td.png)
Replaicing with [A]0 = 0.304M and k = 0.05min-1