Answer:
You need to weight 6,005 g of acetic acid
Step-by-step explanation:
Using Henderson-Hasselbalch formula you will obtain:
5,00 = 4,76 +log₁₀
![([Ac^-])/([Acac])](https://img.qammunity.org/2020/formulas/chemistry/college/346cemj0guf2v0h8mxnncqyfm5a654ot11.png)
Where Ac⁻ is the salt of acetic acid (Acac).
Solving:
1,738 =
![([Ac^-])/([Acac])](https://img.qammunity.org/2020/formulas/chemistry/college/346cemj0guf2v0h8mxnncqyfm5a654ot11.png) (1)
(1)
Also, yo know that:
0,200 M = [Ac⁻] + [Acac] (2)
Replacing (2) in (1):
[Acac] = 0,0730 M.
Thus:
[Ac⁻] = 0,127 M
The moles of each compound are:
Acac = 0,0730 M × 0,500 L = 0,0365 mol
Ac⁻ = 0,127 M × 0,500 L = 0,0635 mol
To prepare these moles it is necessary to use:
Acac + NaOH → AcNa + H₂O
The initial moles of Acac must be:
0,0365 moles + 0,0635 moles = 0,100 moles
To obtain 0,0635 moles of Ac⁻ you need to take this quantity of NaOH moles.
Thus, to obtain a acetate buffer of 5,00 you need to add 0,100 moles of acetic acid and 0,0635 moles of NaOH because This NaOH will react with acetic acid producing 0,0635 moles of Ac⁻ and surplus 0,0365 moles of acetic acid.
Now, to obtain 0,100 moles of acetic acid from pure acetic acid:
0,100 moles ×
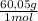 = 6,005 g
= 6,005 g
You need to weight 6,005 g of acetic acid
I hope it helps!