Answer:
None of the statements is incorrect
Step-by-step explanation:
Real gases behave ideally at high pressure and low temperature.
This is due to the increase in intermolecular force of attraction between gaseous molecules at high pressure.
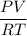 ratio is equal to one for ideal gases where it is greater than or less than 1 for real gases.
ratio is equal to one for ideal gases where it is greater than or less than 1 for real gases.
When the temperature increases, the number gaseous molecules colliding the walls of container will also increase thus increasing the pressure of gas.