Answer: neutral
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7.
![pH=-\log [H^+]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ipfjz05f4cfbguiwup37xvxa7furlbuapf.png)
![pOH=-\log[OH^-]](https://img.qammunity.org/2020/formulas/chemistry/high-school/n477c3o3xy8p6ug3ipfjqh9fd53hdrrjyq.png)
Putting in the values:
![pOH=-\log[10^(-7)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/k18jf98jl9dmogtutkc7s1qbpb034hmaov.png)
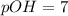
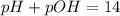
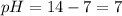
Thus as pH is equal to 7, the solution is neutral and nether accepts
 nor donates
nor donates
 .
.