Step-by-step explanation:
We have given that A 1.05-L bulb and a 1.52-L bulb are connected by a stopcock and filled with argon at 0.72 atm and helium at 1.08 atm respectively, at the same temperature.
Let P₁(p) be the partial pressure of gas in bulb A then
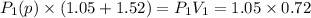
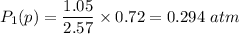
Similarly, Let
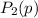 be the partial pressure of gas in bulb B then
be the partial pressure of gas in bulb B then
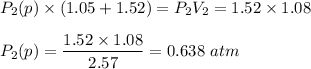
Total pressure= 0.294 + 0.638 = 0.932atm
If n₁ and n₂ be their moles in the respective bulbs
P₁V₁ = n₁ R t
P₂ V₂ = n₂ R t
Now,
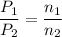
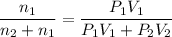
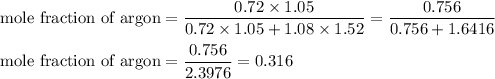

Mole fraction of argon = 0.316
Mole fraction of helium = 0.685