Answer:
1.77mg
Step-by-step explanation:
Hello,
Since the Henry's constant is eligible for
 (hydrogen gas), its concentration into the 315 mL of water is given by:
(hydrogen gas), its concentration into the 315 mL of water is given by:
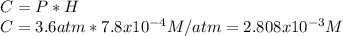
Now, with the following factors, the mass in grams is found as:
![m=(2.808x10^(-3) (mol H_(2))/(L) )*(0.315L)*((2g H_(2) )/(1 mol H_(2)) )*((1000 mg H_(2))/(1 g H_(2)) )</p><p>[tex]m=1.77 mg H_(2)](https://img.qammunity.org/2020/formulas/chemistry/high-school/pmwgibsor29606qg7dvogyz7pvvbioyvll.png)
Taking into account that the first parenthesis accounts for the concentration of
 , the second one for the volume of water, the third one for the molar mass of
, the second one for the volume of water, the third one for the molar mass of
 and the last one for the conversion from g of
and the last one for the conversion from g of
 to mg of
to mg of
 .
.
Best regards.