Answer:
0.44g
Step-by-step explanation:
Given parameters:
Mass of magnesium hydroxide = 0.645g
Solution
The balanced reaction equation is shown below:
Mg(OH)₂ → MgO + H₂O
From the equation above, we see that the number of atoms are conserved.
To find the mass of magnesium oxide produced, we find the number moles of the decomposed magnesium hydroxide first:
Molar mass of Mg(OH)₂ = 24 + 2(16+1) = 58g/mol
Number of moles of Mg(OH)₂ =
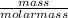
=
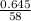
= 0.011mole
Now, from the balanced equaiton, we know that:
1 mole of Mg(OH)₂ produces 1 mole of MgO
Therefore, 0.011mole of Mg(OH)₂ will produce 0.011mole of MgO
so mass of MgO = number of moles of MgO x molar mass of MgO
Molar mass of MgO = 24 + 16 = 40g/mol
mass of MgO = 0.011 x 40 = 0.44g