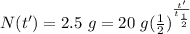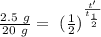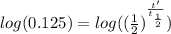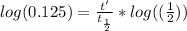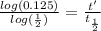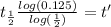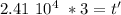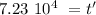Answer:
It will take
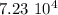 years.
years.
Step-by-step explanation:
Plutonium 239 has a half life of
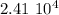 years.
years.
We know, for radioactive decay, that the quantity of radioactive material N at time t can be obtained with the equation
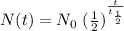
where
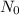 is the initial quantity of radioactive material and
is the initial quantity of radioactive material and
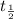 is the half life of the material.
is the half life of the material.
Taking the values of our problem: