The mass, in kg, of Sulfur produced : 2.072 kg
Further explanation
Given
V = 8.56 kL = 8560 L
P = 175 kPa = 1,73 atm
T = 250 + 273 = 523 K
Required
mass of Sulfur produced
Solution
mol of H₂S :

mol of Sulfur based on mol H₂S as a limiting reactant( excess Sulfur dioxide)
From equation, mol ratio H₂S : S = 16 : 3, so mol S :
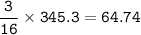
Mass S(Ar = 32 g/mol) :
= mol x Ar s
= 64.74 x 32
= 2071.68 g = 2.072 kg