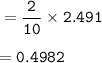Moles of Butane combusted : 0.4982
Further explanation
Given
55.8 water vapour at STP
Required
moles of Butane
Solution
Reaction(Combustion of Butane) :
2C₄H₁₀ + 13O₂ → 8CO₂ + 10H₂O
1 mol at STP = 22.4 L, so mol of H₂O for 55.8 L :
=55.8 : 22.4
=2.491 moles
mol H₂O based on Butane as a limiting reactant(excess Oxygen)
From the equation, mol ratio Butane-C₄H₁₀ : H₂O = 2 : 10, so mol Butane :