Answer: Wavelength of the spectral line for hydrogen that corresponds to an electron falling from the n= 6 level to the n = 3 level is 1100 nm.
It lies in the infra red region.
Step-by-step explanation:

 = Wavelength of radiation
= Wavelength of radiation
E= energy
Using Rydberg's Equation:
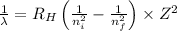
Where,
 = Wavelength of radiation
= Wavelength of radiation
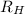 = Rydberg's Constant =
= Rydberg's Constant =
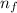 = Higher energy level = 6
= Higher energy level = 6
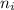 = Lower energy level = 3
= Lower energy level = 3
Z= atomic number = 1 (for hydrogen)
Putting the values, in above equation, we get
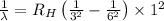
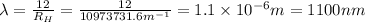
Thus wavelength of the spectral line for hydrogen that corresponds to an electron falling from the n= 6 level to the n = 3 level is 1100 nm.
The wavelength range for gamma rays is less than 0.001 nm, The wavelength range for ultraviolet rays is 1 nm to 400 nm, The wavelength range for visible rays is 400 nm to 750 nm and The wavelength range for infra red rays is 700 nm to 1000000 nm.
Thus the wavelength of 1100 nm corresponds to infra red region.