Answer:
33.67 mg

Step-by-step explanation:
Hello,
Since the Henry's constant allows us to know the concentration of the dissolved
 , we apply the formula:
, we apply the formula:
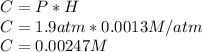
Now, by knowing the concentration and that the volume is 0.426 L of water (proper units), we apply the following factors to know the milligrams of oxygen gas:
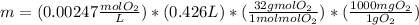
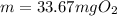
Best regards!