Answer:
8.4 ml of water is needed to make a 10 ml of a 2x concentration.
Step-by-step explanation:
From question,
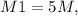
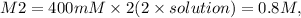
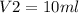
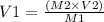
This gives
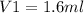
V1 is the available volume of water in the stock solution.
To obtain 10ml of 2x concentration, the volume of water required is:
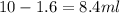 of water.
of water.
Hence 8.4 ml of water is needed to make a 10 ml of a 2x concentration.