Answer: aluminium, mercury , platinum and copper.
Step-by-step explanation:
A single replacement reaction is one in which a more reactive element displaces a less reactive element from its salt solution. Thus one element should be different from another element.
A general single displacement reaction can be represented as :
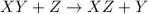
Thus the metals which are more reactive than magnesium will react with magnesium sulphate solution.
The metals which are more reactive than magnesium are aluminium, mercury , platinum and copper.