Answer:
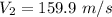
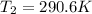
Step-by-step explanation:
At initial condition
P=2 MPa
T=30°C
V=25 m/s
At final condition
P=0.3 MPa
Now from first law for open system
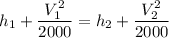
We know that for air
h= 1.010 x T KJ/kg
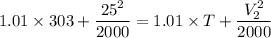 -----1
-----1
Now from mass balance
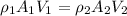
We also know that
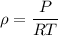
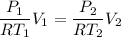
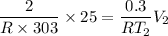
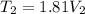 ----------2
----------2
Now from equation 1 and 2
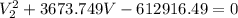
So we can say that
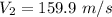
This is the outlet velocity.
Now by putting the values in equation 2
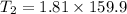
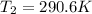
This is the outlet temperature.