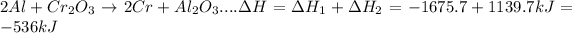Answer:
Change in enthalpy for the reaction is -536 kJ
Step-by-step explanation:
- Overall chemical reaction can be represented a summation of two given elementary steps with slight modification.
- Take reaction (1a) and divide stoichiometric coefficients by 2
- Take reverse reaction (2a) and divide stoichiometric coefficient by 2
- Then add these two modified elementary steps to get overall chemical reaction
 is an additive property. hence value of
is an additive property. hence value of
 will be changed in accordance with modification
will be changed in accordance with modification
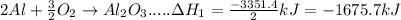
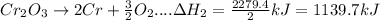
--------------------------------------------------------------------------------------------------------------