Answer:
0.6410g of HgS (mercury (II) sulfide) are formed
Step-by-step explanation:
First you should write the balanced chemical equation, so we have:
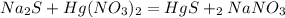
Where:
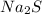 is the formula for the sodium sulfide
is the formula for the sodium sulfide
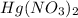 is the formula for the mercury (II) nitrate
is the formula for the mercury (II) nitrate
 is the formula for the mercury (II) sulfide
is the formula for the mercury (II) sulfide
and
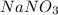 is the formula for the sodium nitrate
is the formula for the sodium nitrate
Then you should calculate the amount of each substance in each solution, so:
- For the
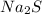 :
:
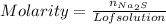
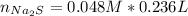
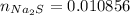 moles of
moles of
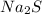
- For the
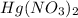 :
:
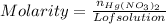
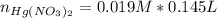
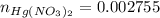 moles of
moles of
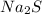
As the quantity of
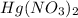 is smaller than the quantity of
is smaller than the quantity of
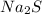 . The
. The
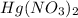 is the limiting reagent and you should work with this quantity, so we have:
is the limiting reagent and you should work with this quantity, so we have:
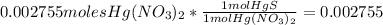 moles of HgS
moles of HgS
And as the molar mass of the HgS is
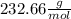 you can calculate the mass of HgS that is produced:
you can calculate the mass of HgS that is produced:
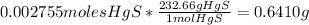 HgS
HgS