Answer:
The inter molecular interactions that occur in benzyl alcohol are hydrogen bonding, dipole-dipole interaction and Van der Waal’s dispersion forces.
Step-by-step explanation:
Benzyl alcohol has the formula
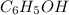 . a hydrogen bond is formed between a hydrogen atom and an electro negative element like fluorine, chlorine, oxygen etc.
. a hydrogen bond is formed between a hydrogen atom and an electro negative element like fluorine, chlorine, oxygen etc.
Benzyl alcohol has a lone pair of oxygen atom which is electro negative. Thus this lone pair bonds with the hydrogen of other benzyl alcohol molecules.
Since
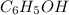 is a polar molecule it has a positive and negative end. Interaction between these ends of opposite polarity is dipole-dipole interaction.
is a polar molecule it has a positive and negative end. Interaction between these ends of opposite polarity is dipole-dipole interaction.