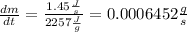Answer:
The rate at which the container is losing water is 0.0006418 g/s.
Step-by-step explanation:
- Under the assumption that the can is a closed system, the conservation law applied to the system would be:
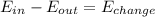 , where
, where
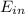 is all energy entering the system,
is all energy entering the system,
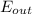 is the total energy leaving the system and,
is the total energy leaving the system and,
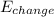 is the change of energy of the system.
is the change of energy of the system. - As the purpose is to kept the beverage can at constant temperature, the change of energy (
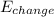 ) would be 0.
) would be 0. - The energy that goes into the system, is the heat transfer by radiation from the environment to the top and side surfaces of the can. This kind of transfer is described by:
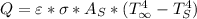 where
where
 is the emissivity of the surface,
is the emissivity of the surface,
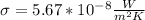 known as the Stefan–Boltzmann constant,
known as the Stefan–Boltzmann constant,
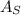 is the total area of the exposed surface,
is the total area of the exposed surface,
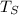 is the temperature of the surface in Kelvin,
is the temperature of the surface in Kelvin,
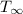 is the environment temperature in Kelvin.
is the environment temperature in Kelvin. - For the can the surface area would be ta sum of the top and the sides. The area of the top would be
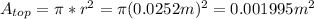 , the area of the sides would be
, the area of the sides would be
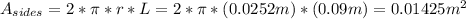 . Then the total area would be
. Then the total area would be
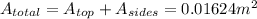
- Then the radiation heat transferred to the can would be
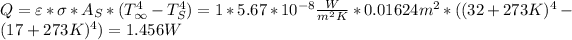 .
. - The can would lost heat evaporating water, in this case would be
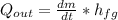 , where
, where
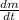 is the rate of mass of water evaporated and,
is the rate of mass of water evaporated and,
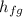 is the heat of vaporization of the water (
is the heat of vaporization of the water (
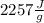 ).
). - Then in the conservation balance:
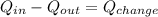 , it would be
, it would be
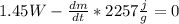 .
. - Recall that
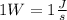 , then solving for
, then solving for
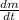 :
: