Answer : The concentration of
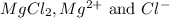 are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.
are 0.127 ppm, 0.127 ppm and 0.254 ppm respectively.
Explanation : Given,
Mass of
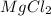 =
=
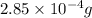
Volume of solution = 2.25 L
First we have to calculate the concentration in terms of g/L.
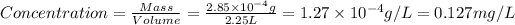
conversion used :
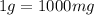
As we know that,
1 mg/L = 1 ppm
0.127 mg/L = 0.127 ppm
So, the concentration will be 0.127 ppm.
The concentration of
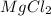 in ppm is, 0.127 ppm.
in ppm is, 0.127 ppm.
Now we have to calculate the concentration of
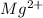 and
and
 .
.
We assume that,
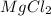 dissociates 100 % in the solution then the balanced reaction will be:
dissociates 100 % in the solution then the balanced reaction will be:
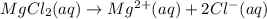
From the reaction we conclude that the mole ratio of
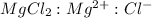 is, 1 : 1 : 2. So,
is, 1 : 1 : 2. So,
The concentration of
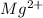 = 0.127 ppm
= 0.127 ppm
The concentration of
 = 2 × 0.127 ppm = 0.254 ppm
= 2 × 0.127 ppm = 0.254 ppm