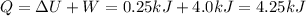Answer:
Work: 4.0 kJ, heat: 4.25 kJ
Step-by-step explanation:
For a gas transformation at constant pressure, the work done by the gas is given by
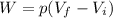
where in this case we have:
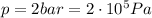 is the pressure
is the pressure
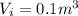 is the initial volume
is the initial volume
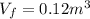 is the final volume
is the final volume
Substituting,
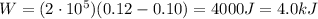
The 1st law of thermodynamics also states that
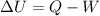
where
 is the change in internal energy of the gas
is the change in internal energy of the gas
Q is the heat absorbed by the gas
Here we know that
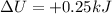
Therefore we can re-arrange the equation to find the heat absorbed by the gas: