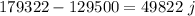Answer:
49822 joules of heat can be transferred to ankle from heat bag.
Step-by-step explanation:
We know that average body temperature should be 37 degree centigrade
Hot water bag has 715 grams of water present in it. Specific heat of 1 gram of water is 4.18 joules per gram per degree centigrade. 715 grams will have
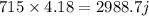
The degree of water that is given is 60° hence specific heat becomes
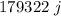
Specific heat of body at 37° centigrade will be =
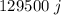
The amount of heat that can be transferred from hot bag will be