Answer:
4 atoms of phosphorus are there in one phosphorus molecule.
Step-by-step explanation:
Density of phosphorus gas,d = 2.7 g/L
Temperature of the gas = T = 280°C = 553.15 K
Molar mass of phosphorus gas = M
Pressure of the gas ,P= 1 atm
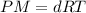
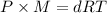 ...(1)
...(1)
Density of nitrogen gas,d' = 0.62 g/L
Temperature of the gas = T'= 280°C = 553.15 K
Molar mass of nitrogen gas = M' = 28 g/mol
Pressure of the gas ,P' = 1 atm
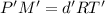
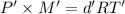 ...(2)
...(2)
Dividing (1) and (2):
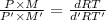
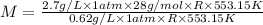
M = 121.93 g/mol
Atomic mass of 1 phosphorus atom = 31 g/mol
Number of phosphorus atom in phosphorus gas:
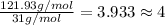
4 atoms of phosphorus are there in one phosphorus molecule.