Answer:
7.7ppm
7700 ppb
Step-by-step explanation:
- To calculate ppm, you need to convert your 258 μg to mg and your 0.0335L to kg using the density of the water:
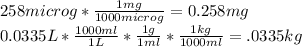
- Now you use the ppm formula to obtain the answer:
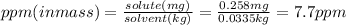
- To calculate ppb you need to consider that ppm is defined as
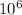 and ppm as
and ppm as
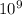 , this means that ppm*1000=ppb
, this means that ppm*1000=ppb
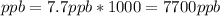
I hope you find this information useful! Good luck!