Answer:
39.42g
Step-by-step explanation:
Given parameters:
volume of the solution = 35.2mL
density of solution = 1.12g/cm³
Unknown:
Mass of solution = ?
Solution:
Density is given as mass per unit volume. It is the amount of substane contained in a solution.
It is mathematically expressed as:
Density =

The dimensional equivalence of mass is designated as M and volume is expressed in lengths, L
Density =
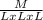
the dimensional equivalence of length is ML⁻³
Therefore, to find the mass of the solution:
Mass = Density x volume
We must express the volume in form of cm³
1mL = 1cm³
so 35.2mL of the solution is the same as 35.2cm³
Mass = 1.12g/cm³ x 35.2cm³ = 39.42g