Answer:
823337.9 J
Step-by-step explanation:
At 1atm of pressure, water's melting point is about 0°C and its vaporization point is 100°C.
(1) At -47°C, water will be in solid state. The heat that is required to warm the solid sample to its melting point is given by:
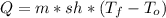
Where sh is specific heat at solid, that tells us the heat per unit of mass required to increase the temperature 1°C at solid state:
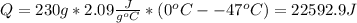
(2) The heat is required to melt the sample is given by:
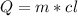
Where cl is the heat of fusion, that tells us the heat per unit of mass to fuse or freeze water.
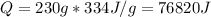
(3) The heat that is required to warm the liquid sample to its boiling point is given by:
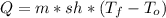
Where sh is specific heat at liquid. that tells us the heat per unit of mass required to increase the temperature 1°C at liquid state:
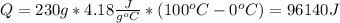
(4) The heat that is required to vaporize the sample is given by:
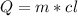
Where cl is the heat of vaporization, that tells us the heat per unit of mass to vaporize or condense water.
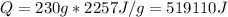
(5) The heat that is required to warm the gas sample to its final temperatur is given by:
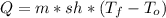
Where sh is specific heat at gas. that tells us the heat per unit of mass required to increase the temperature 1°C at gas:
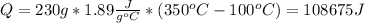
(6)The heat that is required for the entire process to occur is simply the addition of all the heats calculated:
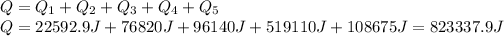
or 823.34 kJ