Answer:
The density of the liquid is 4.9 g/ml
Step-by-step explanation:
Density of a liquid is usually measured by filling a beaker of known weight and known capacity (total volume in ml you can fill it) and weighing it. The difference between the total weight (beaker+liquid) and the beaker weight is the liquid weight. Finally, you must divide liquid weight/volume to obtain density.
For the problem:
beaker=15 g
beaker+liquid=64 g
⇒liquid= 64 g - 15 g = 49 g
density=
 =
=
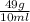 = 4.9 g/ml
= 4.9 g/ml