Answer:
1) Manganese(II)chloride :
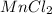
2) Manganese(IV)chloride :
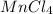
3) Manganese(II)oxide :

4) Manganese(IV)oxide :

Step-by-step explanation:
It is given that:
Oxidation number of chlorine (Cl) = -1
Oxidation number of Oxygen (O) = -2
1) Manganese(II)chloride :
The oxidation number of manganese (Mn) is indicated by the roman numeral II = +2
Oxidation number of chlorine (Cl) = -1
Since the molecule is neutral the sum of charges on the cation (Mn) and anion (Cl) should be zero. This is possible only if one mole Mn combines with 2 moles Cl. Therefore, the chemical formula is
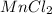
2) Manganese(IV)chloride :
The oxidation number of manganese (Mn) is indicated by the roman numeral IV = +4
Oxidation number of chlorine (Cl) = -1
Since the molecule is neutral the sum of charges on the cation (Mn) and anion (Cl) should be zero. This is possible only if one mole Mn combines with 4 moles Cl. Therefore, the chemical formula is
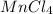
3) Manganese(II)oxide :
The oxidation number of manganese (Mn) is indicated by the roman numeral II = +2
Oxidation number of Oxygen (O) = -2
Since the molecule is neutral the sum of charges on the cation (Mn) and anion (O) should be zero. This is possible only if one mole Mn combines with 1 mole O. Therefore, the chemical formula is

4) Manganese(IV)oxide :
The oxidation number of manganese (Mn) is indicated by the roman numeral IV = +4
Oxidation number of Oxygen (O) = -2
Since the molecule is neutral the sum of charges on the cation (Mn) and anion (O) should be zero. This is possible only if one mole Mn combines with 2 moles O. Therefore, the chemical formula is
