Step-by-step explanation:
A chemical reaction in which heat energy is absorbed by the reactant molecules is known as an endothermic reaction.
For example,
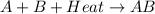
The value of
 = +ve for endothermic reaction.
= +ve for endothermic reaction.
Whereas a chemical reaction in which heat energy is released by the reactant molecules is known as an exothermic reaction.
For example,
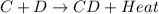 is an exothermic reaction.
is an exothermic reaction.
The value of
 = -ve for exothermic reaction.
= -ve for exothermic reaction.
Also, the given reaction,
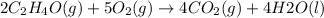
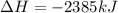
It is also an exothermic reaction because heat is releasing as value of
 is negative.
is negative.
Thus, we can conclude that the given reaction is exothermic in nature.