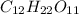Answer:
 >
>
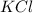 >
>
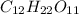
Step-by-step explanation:
Osmotic pressure is a colligative property which depends on the amount of solute added.
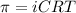
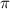 = osmotic pressure
= osmotic pressure
i= vant hoff factor
C= concentration in Molarity
R= solution constant
T= temperature in Kelvin
1.. For

, i= 3 as it is a electrolyte and dissociate to give 3 ions.
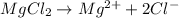
2. For
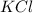
, i= 2 as it is a electrolyte and dissociate to give 2 ions.
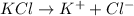
3. For
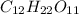
, i= 1 as it is a non electrolyte and does not dissociate.
Thus as vant hoff factor is highest for
 , the osmotic pressure will be highest.
, the osmotic pressure will be highest.
The order from highest to lowest osmotic pressure will be :
 ,
,
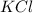 and
and