Answer:
The density of the crown is 10,71 g/mL. Thus, the crown is not pure gold.
Step-by-step explanation:
As volume of container is 1900 the mass of water when filled to the mark is:
1900,0mL×
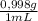 = 1896,2 g of water
= 1896,2 g of water
The mass of 2499,5 g is the mass of container with water. As the mass of water is 1896,2 g the mass of container is:
2499,5 g - 1896,2 g = 603,3 g -container-
When the crown is placed in the container and this is filled with water the mass of this water is:
15113,3 g - 13910,0 g - 603,3 g = 600 g of water
Total mass - crown - container
This 600,0 g occupy a volume of:
600,0 g×
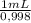 = 601,2 mL
= 601,2 mL
Thus, volume of the crown is:
1900,0 mL - 601,2 mL = 1298,8 mL
Total volume - Water
The density of the crown is:
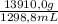 = 10,71 g/mL
= 10,71 g/mL
As density of the crown is different to density of pure gold, the crown is not made of pure gold.
I hope it helps!