Answer:
1) 19.739 kmol/min
2) v = 0.021 m/s
D = 12.788 m
Step-by-step explanation:
1) By the mass balance, the amount that enters a system such be the amount the goes out, if there isn't accumulation.
Knowing that the molar masses are: C = 12 kg/kmol and O = 16 kg/kmol; CO₂ has molar mass equal to: 12 +2x16 = 44 kg/kmol. So, the initial flow rate of CO₂ is
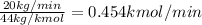
Which is the same of the upstream. So, if the concentration of CO₂ is 2.3 % or 0.023, than the flow rate of the gas(F) will be:
0.023F = 0.454
F = 19.739 kmol/min
2) First, let's estimate the velocity of the gas. The pipeline has 10 meters, and the flow occurs during 8 min = 480 s, so the velocity is:
v = 10/480 = 0.021 m/s
For a flow of 19.739 kmol/min during 8 min, the number of moles of the gas will be:
n = 19.739x8 =157.912 kmol
The density is the number of moles divided by the volume, so:
0.123 = 157.912/V
V = 157.912/0.123
V = 1283.837 m³
The pipeline is a cylinder, so the volume is:
V = πxr²xH
Where π = 3.14, r is the radius and H the height of the pipeline = 10 m
1283.837 = 3.14xr²x10
r² = 40.886
r = √40.866
r = 6.394 m
The diameter is two radius, so
D = 12.788 m