Chlorine:
Atomic Number = 17
Atomic Mass = 35.453
Amount of Protons = 17
Amount of Neutrons = 18
Amount of Electrons = 17
Therefore your answer is option A "17." You can find the number of electrons by looking at the elements atomic number which is equal to the amount of protons the element has then the protons is equal to the amount the electrons it has so....
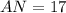
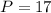
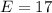
Hope this helps.