Answer:
1178 inches
Explanation:
Using the triangle I drew (assuming n is across from ∠N), we can conclude that ∠O = 180-97-32 = 51 as the angles in a triangle add up to 180.
Using the law of sines, we can conclude that
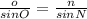
Plugging our values in, we get
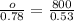
Multiply both sides by 0.78 to get
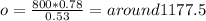
round up to get 1178 as our answer