Answer:
C is the fastest reaction.
Step-by-step explanation:
Let us consider a generic reaction aA ⇄ bB.
The reaction rate would be:
![r=k.[A]^(n)](https://img.qammunity.org/2020/formulas/chemistry/college/oqobk28r7q49sci1nuqpc42nst6re9cner.png) [1]
[1]
where,
r is the reaction rate
k is the rate constant
n is the reaction order
So we can see that the reaction rate depends on the rate constant. We can find out this constant using the Arrhenius equation:
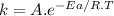 [2]
[2]
where,
A is the collision factor
R is the universal gas constant
T is the absolute temperature
If A and T are the same for different reactions, k will only depend on Ea. According to [2], the lower Ea, the higher the k. According to [1], the higher the k, the higher the r. And when r is higher the reaction is faster. All in all, C has the lowest Ea (34 kJ/mol), so it is the fastest reaction.