Answer:
a) 1,34 Fe(s) moles and 1,25 O₂(g) moles.
b) Limiting reagent is Fe(s).
c) 0,67 Fe₂O₃ moles
Step-by-step explanation:
a) The first thing we should know is how many moles are of each reagent, thus:
- 75,0 Fe(s) grams ×
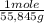 = 1,34 Fe(s) moles
= 1,34 Fe(s) moles
-Using atomic mass of Iron-
- To obtain O₂ moles it is necessary to use ideal gas law:
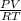 = n
= n
Where:
P is pressure: 2,66 atm
V is volume: 11,5 L
R is gas constant: 0,082
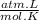
T is temperature: 298 K
n are unknowed moles.
Thus, moles are: 1,25 O₂ moles
b) The global reaction is:
4 Fe(s) + 3 O₂(g) → 2 Fe₂O₃
So, four Fe(s) moles reacts with three O₂(g) moles. It means that for a complete reaction of 1,34 Fe(s) moles you need:
1,34 Fe(s) moles ×
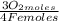 = 1,01 O₂ moles
= 1,01 O₂ moles
For a complete reaction of this iron you need just 1,01 O₂ moles but you have 1,25 O₂ moles. Thus, limiting reagent is Fe(s).
c) Now, the produced Fe₂O₃ moles are calculated with the limiting reagent moles, knowing that 4 Fe(s) moles produce 2 Fe₂O₃ moles -global reaction-, thus:
1,34 Fe(s) moles ×
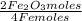 =
=
0,67 Fe₂O₃ moles
I hope it helps!