Answer:
The answer is for a) 210 mL of ethanol b) 75 mL of water.
Step-by-step explanation:
When we prepare a solution using a stock solution we use the following equation:
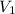 ×
×
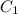 =
=
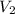 ×
×
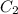
This is an equation for dilutions and we can use it in the case of our answer.
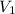 = is our unknown value but we know the other three values in the equation:
= is our unknown value but we know the other three values in the equation:
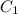 = 95%
= 95%
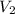 = 285 mL
= 285 mL
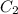 = 70%
= 70%
So, clearing the previous given formula we obtain:
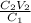 =
=
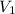
and replacing for numerical values:
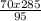 = 210 mL this is the value of ethanol stock solution we need to use
= 210 mL this is the value of ethanol stock solution we need to use
and subtracting this value of ethanol from the total volume of the solution
285 mL - 210mL = 75 mL of water needed.