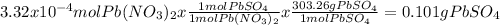Answer:
0.101g PbSO₄
Step-by-step explanation:
To know an amount of a product we have to know first how many moles of reactants we have.
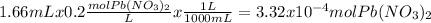
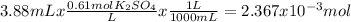
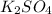
The reaction is:
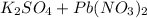 ⇒
⇒
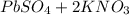
We can observe that per one mole of K₂SO₄ we need one mole of Pb(NO₃)₂, so Pb(NO₃)₂ is the limitant reactant and reaction will stop when it´s over.