Answer:
52.28g
Step-by-step explanation:
Given:
Mass of CO₂ = 71.89g
Solution:
The equation of the reaction:
CO₂ → C + O₂
We want to determine the mass of O₂ that will form from this reaction.
To do this, find the number of moles of CO₂:
Number of moles of CO₂ =
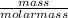
Molar mass of CO₂ = 12+ 2(16) = 44g/mol
Number of moles of CO₂ =
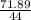 = 1.63mole
= 1.63mole
From the equation of the reaction:
1mole of CO₂ will produce 1 mole of O₂
So 1.63 mole of CO₂ also produces 1.63mole of O₂
The mass of O₂:
Mass of O₂ = number of moles of O₂ x molar mass of O₂
Molar mass of O₂ = 2 x 16 = 32g/mol
Mass of O₂ = 1.63 x 32 = 52.28g