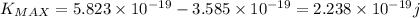Answer:
Max kinetic energy for 340 nm wavelength will be
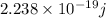
Step-by-step explanation:
In first case wavelength of electromagnetic radiation
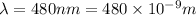
Plank's constant
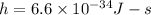
Maximum kinetic energy = 0.54 eV
Energy is given by
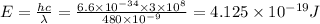
We know that energy is given
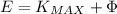 , here
, here
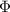 is work function
is work function
So
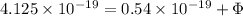
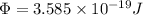
Now wavelength of second radiation = 340 nm
So energy
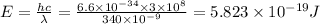
So