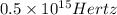Answer: Frequency corresponding to a photon possessing this energy is
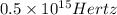
Wavelength for this photon is 600 nm.
Step-by-step explanation:
The relationship between wavelength and energy of the wave follows the equation:

E= energy
 = wavelength of the wave
= wavelength of the wave
h = Planck's constant =
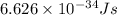
c = speed of light =
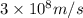
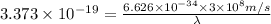
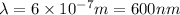
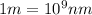
Thus wavelength for this photon is 600 nm.
The relationship between wavelength and frequency of the wave follows the equation:
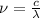
where,
 = frequency of the wave
= frequency of the wave
c = speed of light
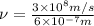
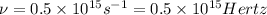
Thus frequency corresponding to a photon possessing this energy is