Answer: Option (C) is the correct answer.
Step-by-step explanation:
Movement of particles in a substance is responsible for change in state of the substance or matter.
This means that more is the motion of particles more will be their kinetic energy.
Also, kinetic energy is directly proportional to temperature.
K.E =
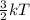
So, less is the temperature of an object or substance less will be be the motion of its particles. Therefore, molecules will come closer to each other and state of substance will change from liquid to solid.
Thus, we can conclude that the motion of the molecules would decrease at a molecular level if a liquid is placed in cool conditions.