Answer:
A strong acid has a weak conjugate base;
A Brønsted base is a proton acceptor;
Bases need not contain –OH directly; instead, they can increase the concentration of –OH by deprotonating water.
Step-by-step explanation:
Acetic acid (CH3COOH) is an oxyacid, and it's deprotonating occurs in equilibrium. The equilibrium constant for this acid is Ka =
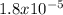 , pKa will be
, pKa will be
pKa = -logKa = 4.74
So, for higher values of pKa, less strong is the acid.
For the theory of conjugation seesaw, it must have been an equilibrium if the acid and it's the conjugate base. A strong acid has a weak conjugate base, and a weak acid has a strong conjugate base.
For the theory of Brønsted-Lowry, an acid is a proton donator, and a base is a proton acceptor, but they don't need to have
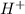 and
and
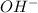 directly, they can increase their concentration by deprotonating water.
directly, they can increase their concentration by deprotonating water.