Answer:
[
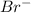 ]=0.4M
]=0.4M
Step-by-step explanation:
When you add bromide to a silver nitrate solution, silver bromide can be produced. It depends of the reactants concentration.
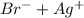 ⇄
⇄
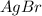 Kps for this reaction is
Kps for this reaction is
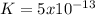 , this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.
, this constant indicates if the reaction can happen spontaneously or not. in this case, a small value of Kps means that precipitate can be formed.
Now we need to know if our conditions are enough to form that precipitate.
![[Br^(-)]=6.54gKBr.(1molKBr)/(119gKBr).(1molBr^(-) )/(1molKBr) .(1)/(0.05L) =1.10M](https://img.qammunity.org/2020/formulas/chemistry/high-school/12wm271w2hh9yck0g9aaxmczoroey3zn2u.png)
![Qps=[Br^(-)][Ag^(+)]](https://img.qammunity.org/2020/formulas/chemistry/high-school/iu7veui4b73s356sewfbf7u92nn1hauzug.png) =
=
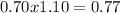
Due to Qsp>Ksp precipitate will be formed. notice that reaction will go on until silver has been consumed completely, silver is limiting reagent.
moles of bromide remainig =total moles of bromide-moles of bromide that reacts
moles of bromide that reacts are the same number of moles from silver beacause in reaction the mole ratio is 1:1.
![[Br^(-)]=(((1.1mol)/(L).0.05L-(0.70mol)/(L).0.05L)/(0.05L) =0.4M](https://img.qammunity.org/2020/formulas/chemistry/high-school/u1kvh0d5mopf4u1276nte1e2yo6osdrk0m.png)