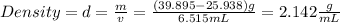Answer:
Density=2.142 g/mL
Step-by-step explanation:
Density is defined as the mass per unit volume of a compound, the mass of the NaCl solution is given by the subtraction of the mass of the cylinder with the solution minus the mass of the empty dry cylinder.